

Higher and foundation tiers
 Potable water is water that is
safe to drink. In the UK we tend to take it for granted that when we turn on the tap
fresh clean water will always be available. For many millions of people around the world this is simply not the case. Most of
our potable water comes from surface water sources such as rivers, lakes and reservoirs which are filled by
rain water. However water from these
sources is unlikely to be fit to drink due to the high levels of
bacteria, viruses and other harmful pathogens that may be present in it; this water will need further treatment before
it is fit to drink.
Potable water is water that is
safe to drink. In the UK we tend to take it for granted that when we turn on the tap
fresh clean water will always be available. For many millions of people around the world this is simply not the case. Most of
our potable water comes from surface water sources such as rivers, lakes and reservoirs which are filled by
rain water. However water from these
sources is unlikely to be fit to drink due to the high levels of
bacteria, viruses and other harmful pathogens that may be present in it; this water will need further treatment before
it is fit to drink.
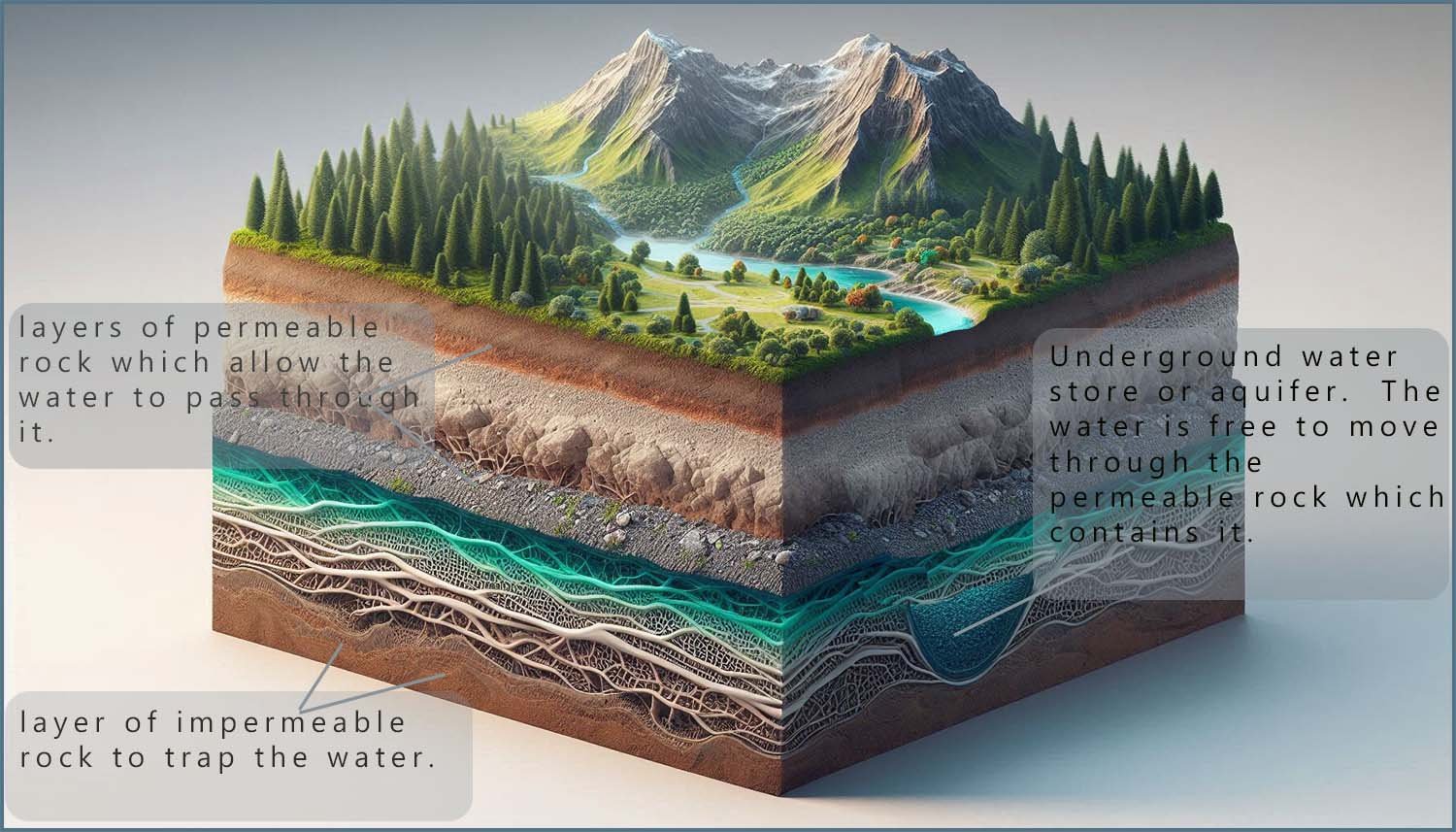
However not all water sources are on the surface; there are many sources of underground water such as water which is stored in underground aquifers. An aquifer is a layer of rock or sediment that stores groundwater. Aquifers are important because they provide a significant source of water for many communities. Wells can be drilled into underground aquifers to extract groundwater for drinking, irrigation and other uses.
Once rain water
and other sources of ground water soak into the Earth it will sink and can become trapped in layers of permeable rocks. These are rocks
which are porous and act a
little like a sponge allowing the water to move through pores and holes in the
rock. These underground water stores are referred to as aquifers. These aquifers can be
huge and cover large areas of land.
Water taken from these underground
aquifers via boreholes and wells is not potable even though it has been filtered
by the rock and other layers it passes through, it still needs
to be disinfected with chlorine or other sterilising agents before it is fit to drink.
The groundwater found in these aquifers can also be contaminated with various industrial and agricultural pollutants, including nitrates, heavy metals, pesticides, and other chemicals. The type of pollution present will depend mainly on the land use practices in the area. Therefore, the water may require testing and treatment beyond just simple disinfection. The image below shows a cross-section of land which contains an aquifer:
Water from rivers and reservoirs may contain larger particles of mud and sand which may discolour the water. There may be larger objects such as twigs, branches and of course a shopping trolley as well as other rubbish and organic material such as dead plants and animals! There will also be bacteria, viruses and other microbes present in the water which could cause various illnesses if someone drank this water.
As the rainwater collects in rivers, streams, reservoirs and underground aquifers then it may pass over rocks which contain soluble minerals that will dissolve in the water. Hard water, for example, is simply water that contains relatively higher concentrations of calcium and magnesium ions. While the hardness of water is sometimes seen as a nuisance due to the build up of limescale in kettles, showers and heating systems and formation of soap scum, it can have health benefits by providing essential nutrients. There are some studies that suggest that consumption of water with sufficiently high levels of calcium and magnesium ions is associated with lower rates of cardiovascular diseases, osteoporosis, and metabolic disorders, however the evidence is not universally conclusive.
While minerals such as calcium and magnesium ions which are present in hard water can have health benefits, other substances found in drinking water can be harmful. Fertilisers, pesticides and herbicides washed off farmers’ fields, for example, can enter rivers and lakes, where they may harm aquatic life and pose risks to human health. These pollutants can also be very difficult to remove during water treatment.
The main pollutants washed off farmland include nitrates, phosphates, pesticides and bacteria. Nitrates from fertilisers can cause health problems if present in high enough concentrations, especially for babies, and can also lead to eutrophication in rivers and lakes. Phosphates from fertilisers and manure contribute to eutrophication too, encouraging rapid algal growth that depletes oxygen levels in the water. Pesticides and herbicides used to control pests and weeds can also contaminate groundwater and are difficult to remove during water treatment. Slurry and animal waste from livestock farms may introduce bacteria such as E. coli and other pathogens, making the water unsafe to drink.

Complete the activity below by simply matching the pollutant to the unwanted effects it has on the water quality and human health.
Click a pollutant on the left, then click an effect on the right to match them. When you have matched them all, press “Check answers”.
1. Pollutants (from farmland):
2. Effects on water and health:
Tip: Click an effect with no pollutant selected to clear its match.
The image below gives an indication of some of the processes that take place at the waterworks to remove these harmful pollutants and harmful disease causing pathogens from the water and make it fit to drink.
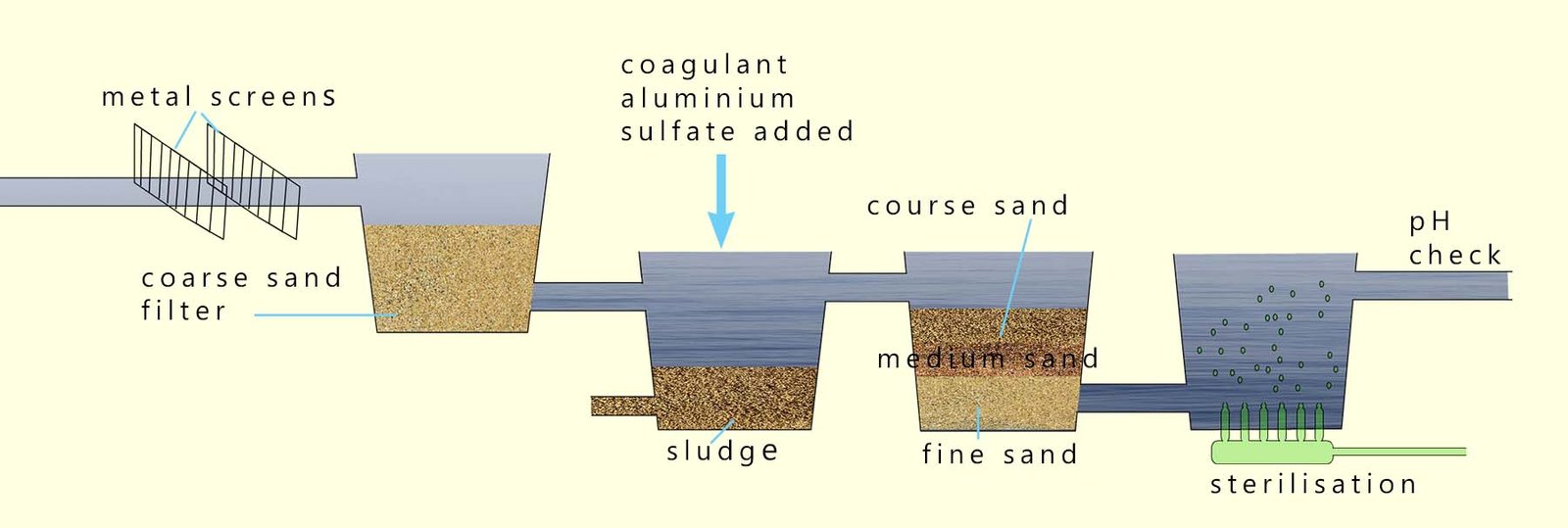
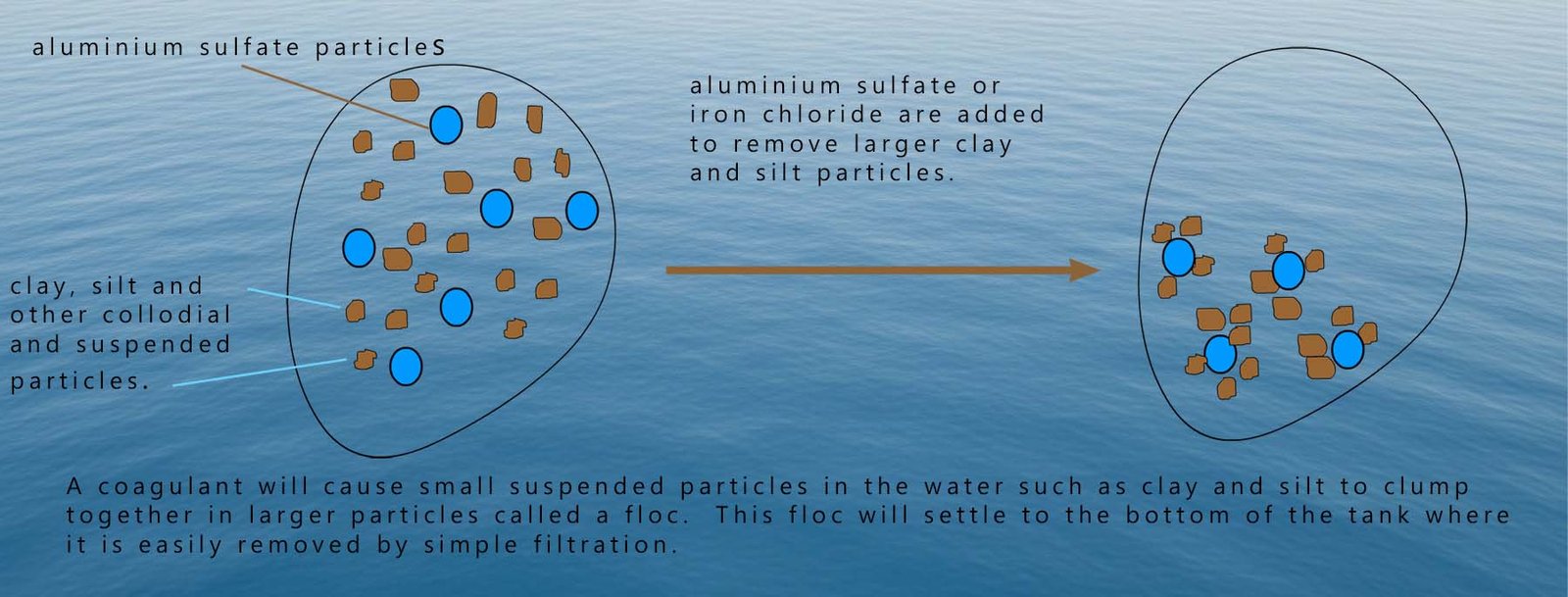
The suspended clay and silt particles present in water after the sedimentation process are often covered in bacteria, viruses and other harmful microbes. After the removal of these particles by filtration through a filter made of fine sand the water is much clearer and cleaner.


Put the statements in the activity below in the correct order to show what happens at the waterworks to produce potable water from dirty water.
Click a stage on the left, then click a box on the right to place it in order (1 is first, 6 is last). When you have filled all 6 boxes, press “Check order”.
1. Choose a stage:
2. Place it in a box:
Tip: Click a filled box to clear it. Click a stage again to deselect it.
In many areas of the world there is not enough fresh water to drink, particularly in the Middle East and North Africa; so how do people living in these hot dry areas get enough water to drink? The answer is they get their fresh water from the sea. Obviously you cannot drink seawater because it contains too much salt; but in a process called desalination the salt is removed from seawater to leave fresh water. There are two basic ways to desalinate seawater; these are:
Distillation is a technique you should already be familiar with; here salt water is simply heated till it boils. The steam produced then enters a Liebig condenser where it cools and condenses and turns back into liquid water. The salt will be left in the round-bottomed flask. The apparatus for simple distillation is shown below:
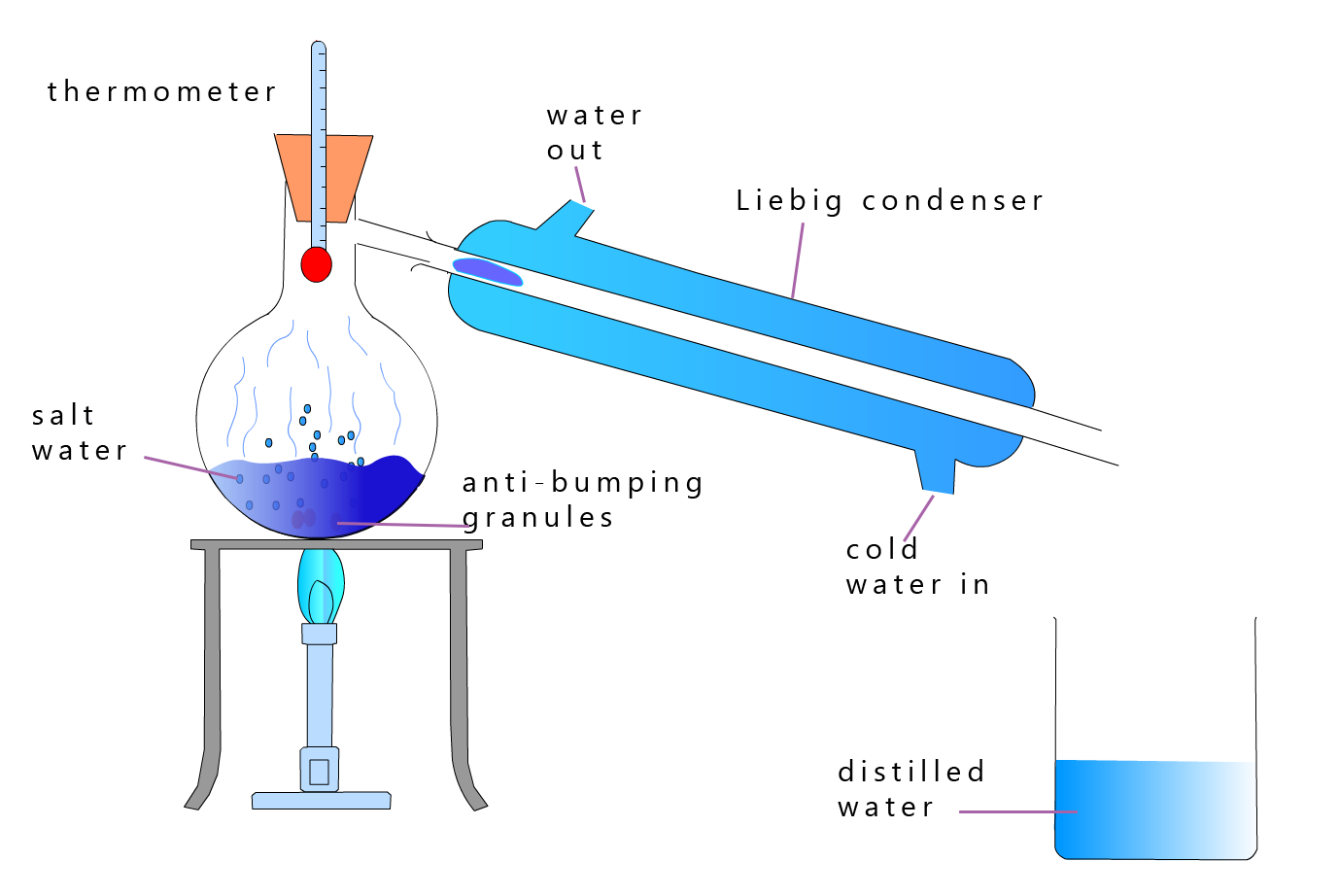
To obtain fresh water from the salt water in a more economical way the salt water can be heated under reduced pressure; this will decrease its boiling point and reduce the amount of energy needed to boil the salt water, however distillation is still a very energy intensive process and is not economic as a method for producing large volumes of fresh water due to the large costs involved. However for some countries such as Saudi Arabia and the UAE this is the only way they can obtain enough fresh water to meet their needs.
Another method used to get fresh water from sea water is called reverse osmosis. Here a high pressure is used to force water molecules through tiny holes in a semi-permeable membrane. These holes will only allow water molecules to travel through and prevent ions which are dissolved in the water from travelling through; this is outlined in the image below:
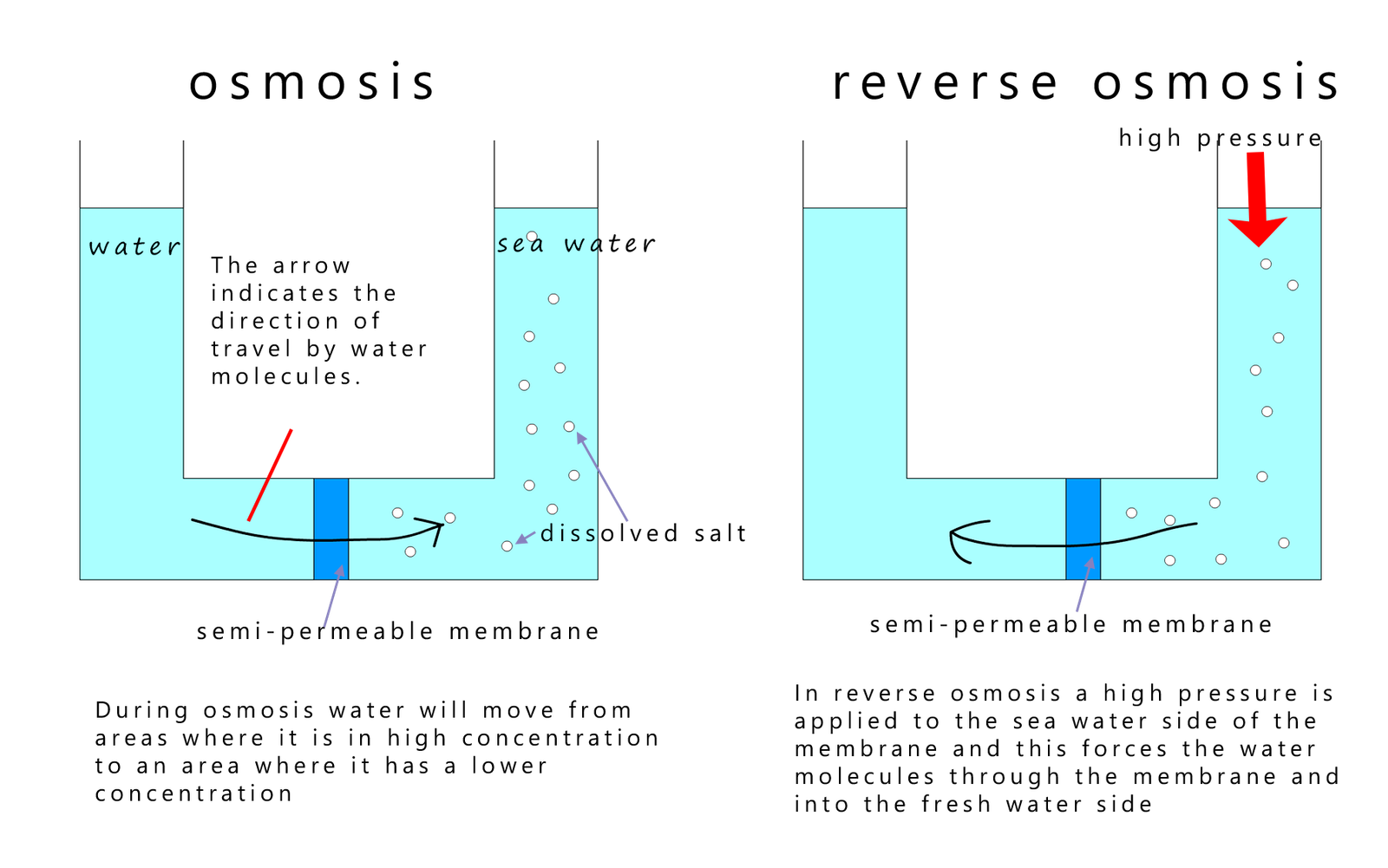
One of the problems, other than cost and the large energy requirements for reverse osmosis to occur, is the fact that a concentrated salt solution called brine is left once fresh water has been removed from the sea water; essentially this process will increase the concentration of the salt in the sea water. This brine solution will then likely be pumped back into the ocean and is likely to harm the local marine environment.